 (1) (1).png)
非洲知识产权组织(Organisation Africaine de la Propriété Intellectuelle,简称OAPI)是一个区域性知识产权组织,由非洲地区17个成员国构成。
该组织成立于1962年9月13日,总部位于喀麦隆雅温得,成员国包括贝宁、布基纳法索、喀麦隆、中非共和国、科摩罗、刚果(布)、科特迪瓦、加蓬、几内亚、几内亚比绍、赤道几内亚、马里、毛里塔尼亚、尼日尔、塞内加尔、乍得和多哥。OAPI依据1999年和2015年修订的《班吉协定》运作,其主要目标是在成员国内建立统一、高效的知识产权保护与执法体系,以推动该地区的创新、创造力和经济发展。此外,OAPI的集中化管理模式确保了申请人及权利持有人能够在各成员国高效、统一地获取并管理其知识产权。
OAPI提供广泛的服务,涵盖专利、商标、外观设计、地理标志等知识产权的注册与保护。通过单一申请程序即可在所有成员国获得知识产权保护,OAPI大大减轻了单一国家申请所涉及的行政负担和成本。
这一体系不仅简化了申请流程,还确保该区域内知识产权法律和标准的一致适用,从而强化了企业和自然人发明人的法律确定性和稳定性。由于所有OAPI成员国均受《班吉协定》统一法律约束,申请人无法选择仅在部分成员国寻求保护。因此,通过OAPI注册的知识产权将在所有成员国同时生效。
除构建区域性知识产权体系外,OAPI还推动多项倡议和国际合作,以提高各方对知识产权重要性的认识并提供相关教育。该组织定期举办培训项目、研讨会和讲习班,旨在提升知识产权从业人员、官员、申请人及公众的专业技能与认知水平。这些举措对维护非洲地区健全的知识产权体系至关重要,为创新保护及后续经济增长奠定了稳定基础。
OAPI还与世界知识产权组织(WIPO)等国际组织合作,确保其利益与实践符合全球最佳标准和法律框架。此外,OAPI是《海牙协定》和《马德里议定书》的缔约方,允许通过该区域性组织进行各类知识产权的国际注册。此类合作为构建高效的区域知识产权体系发挥了重要作用,有助于鼓励全球申请人在OAPI成员国提交知识产权保护申请。
通过吸引外资、鼓励全球知识产权申请,OAPI在推动经济发展中扮演着关键角色。凭借可靠的知识产权保护体系,OAPI为希望挖掘该地区增长潜力的投资者创造了有利环境。由于安全且可执行的知识产权对企业进驻非洲市场至关重要,OAPI的保护机制能有效防止创新成果和品牌标识遭受侵权。此外,促进该区域内知识产权的发展也有助于本土创新的商业化及新产业的培育,从而进一步推动经济增长并增加就业机会。
总体而言,OAPI通过集中化的知识产权体系、统一的法律规范以及知识产权领域的培训支持,推动了17个成员国内的知识产权保护发展。在提供一体化知识产权保护体系的同时,OAPI也成为该地区经济发展的催化剂。
知识产权是现代社会的核心基石,许多流行文化现象的背后都潜藏着知识产权的隐形规则,而大众往往对此浑然不觉。从音乐排行榜作品到玩具品牌,再到其他领域,非传统知识产权中蕴藏着诸多值得探索的经典案例。以下我们将聚焦一些极具代表性的范例。
商标是各行业巨头最重视的知识产权类型之一。尽管多数商标采用传统形式,但部分企业选择另辟蹊径,打造出极具辨识度的非传统商标。
例如,玩具公司孩之宝(Hasbro)于2018年5月15日向美国专利商标局(USPTO)成功注册了其经典产品培乐多(Play-Doh)的气味。
气味商标虽非前所未闻,但因注册门槛较高而较为罕见。以美国为例,法规明确要求“气味或香氛作为商标需提交大量证据”。
此类证据需证明该气味具有显著性且不得是产品的功能性要素,而仅作为品牌识别标识存在。
培乐多的气味在商标注册簿中被描述为“一种甜甜的、略带麝香味、像香草味的香味,带有轻微的樱桃味以及咸味小麦面团的天然气味”。尽管多数人难以通过嗅觉分辨其成分,但这一怀旧气息整体上已具备足够显著性,使消费者能将其与培乐多品牌直接关联。该气味还被制成香水发售,成为极具小众吸引力的纪念品。
相较于商标,版权保护在非传统创作形式中更具复杂性。通常人们将绘画或文学作品视为典型版权作品,但纹身艺术家S·维克多·惠特米尔(S. Victor Whitmill)因专为拳击传奇人物迈克·泰森设计的标志性纹身引发了一场轰动性版权诉讼。该纹身创作于2003年,却在热门电影《宿醉2》(The Hangover II)中出现争议——演员艾德·赫尔姆斯(Ed Helms)饰演的角色面部复刻了泰森的纹身设计。
电影上映前几周(该片最终成为当时票房最高的喜剧电影),惠特米尔就纹身设计使用对华纳兄弟提起版权侵权诉讼。尽管法院未批准其临时禁令申请,但法官认可案件具有法律依据,诉讼程序继续进行。尽管最终双方达成保密和解,但是华纳兄弟在结论出来前几周亦承认曾考虑通过数字技术修改纹身以避免侵权。
此案不仅揭示了数字时代知识产权保护的复杂性,也凸显了版权制度的错综复杂。相较于其他作品(如艺术品或文学作品)在保护方面有很多法律先例,纹身版权保护更具特殊性。
根据英国版权法,纹身设计人享有作品知识产权。如果纹身图像被复制,设计人有权收取版税。这意味着,即便纹身在你身上,但如果是纹身师设计的,那么版权归纹身师所有。但是,如果客户自行设计图案,则版权归客户所有。如果纹身师受委托根据客户创意定制设计,可能产生版权纠纷。此时,可考虑共有权。
尽管保护鲜为人知、非常规知识产权存在风险且需周密考量,但培乐多的气味商标与惠特米尔的纹身版权案表明:突破常规的知识产权布局可能带来巨大回报。如需知识产权保护建议,欢迎联系我们。
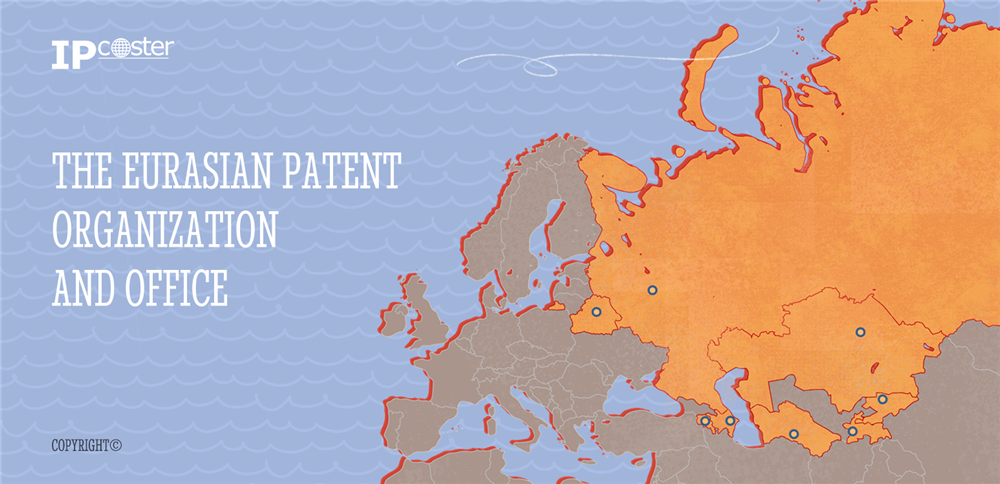
欧亚专利局(EAPO)成立于1995年,是一个政府间组织,以《欧亚专利公约》(EPC)为准则,旨在促进该地区知识产权的保护和发展。EPC于1994年9月9日首次签署,次年8月12日生效,标志着EAPO正式成立。
EAPO的成员国包括独联体国家(CIS)和非独联体国家,有助于促进知识产权领域的国际合作。截至2024年,成员国包括亚美尼亚、阿塞拜疆、白俄罗斯、哈萨克斯坦、吉尔吉斯斯坦、俄罗斯、塔吉克斯坦和土库曼斯坦。EAPO总部位于俄罗斯,官方语言为俄语。
EAPO的主要目标是通过在8个成员国提交一件欧亚申请,推动发明和外观设计相关的知识产权保护。因此,在简化欧亚地区知识产权申请流程中,EAPO起着至关重要的作用,为发明人和企业提供了一个统一的平台,为其同时在多个成员国寻求知识产权的保护提供了可能性。因此,EAPO体系有助于简化申请发明和外观设计保护的流程,削减开支,提高效率,从而促进该地区创新和技术的发展。
欧亚专利局成立初始即致力于保护欧亚专利,并在2019年通过《欧亚专利公约工业品外观设计保护议定书》后,于2021年6月开始受理区域外观设计保护的申请。
如要在EAPO成员国获得欧亚专利,申请人仅需向EAPO提交一件申请,不限语言,但需在之后2个月内递交其俄文译本。如已缴纳相应费用,此期间可延长至4个月。也可通过PCT申请进入欧亚地区获得欧亚专利,应自最早优先权日起31个月内。欧亚专利申请应申请人请求进行实质审查。巴黎公约进欧亚,应自检索报告公布之日起6个月内提交实审请求;PCT进欧亚,应在进入欧亚地区时同时提交实质审查请求。
授权欧亚专利后,专利权人可选择需缴纳维持费的成员国以及恢复因未缴纳维持费而失效的欧亚专利。在部分发明领域,申请人也可要求延长欧亚专利的有效期。自欧亚专利公布之日起至缴纳首次年费期间,专利权人对其发明专利在欧亚地区有独占权。
需直接向EAPO缴纳欧亚发明或外观设计专利相关费用,而非单独缴纳至各个成员国。如提供国际检索报告或由其他国际检索单位(ISA)开具的PCT检索报告,可减缴25%的欧亚专利申请费。如此类报告由俄罗斯联邦知识产权局开具,可减缴40%的申请费。
自2022年起,以《专利合作协定》(PCT)为准则,EAPO成为国际检索单位(ISA)以及国际初审单位(IPEA)。
EAPO积极参加多项国际合作,包括与非洲知识产权组织(OAPI)、非洲地区知识产权组织(ARIPO)、欧盟知识产权局(EUIPO)、欧洲专利局(EPO)以及世界知识产权组织 (WIPO) 签署《谅解备忘录》(MoU's)。
此外,EAPO还与日本专利局(JPO)、韩国知识产权局(KIPO)、芬兰专利注册局(PRH)以及EPO在内的全球多家知识产权局开展专利审查高速路项目。通过与签署PPH协议的各个知识产权局共享审查结果,加快了整个专利申请流程。
EAPO致力于知识产权保护,为该地区的研究、发展和经济增长创造了一个良好的环境。如您想了解在欧亚或通过EAPO申请知识产权保护的更多信息,请通过社交平台联系我们或 点击此处!

欧盟知识产权局(EUIPO)成立于1994年,是一个地区性的知识产权局和欧盟机构,总部位于西班牙阿利坎特。因此,EUIPO致力于保护欧盟商标(EUTM),过去称之为“欧共体商标”,以及注册共同体外观设计(RCD)。EUIPO还有一个法语名称,即Office de l'Union européenne pour la propriété intellectuelle。
EUIPO根据欧洲议会和理事会于2017年6月14日制定的关于欧盟商标2017/1001号条例(EU)成立和运作。作为知识产权相关事务的国际中心,EUIPO使用5种工作语言,即英语、法语、德语、意大利语和西班牙语。EUIPO受理的申请可用欧盟23种官方语言处理。
注册共同体外观设计以及通过EUIPO注册的欧盟商标可在所有欧盟成员国享受统一保护效力。EUIPO会公布注册的商标和外观设计并在所管理的相应知识产权登记簿中披露相关信息。欧盟商标登记簿通常包括所有欧盟商标申请和注册的详细信息,而欧盟外观设计登记簿常含有已注册的欧盟外观设计相关信息。这两个登记簿会持续更新,跟进欧盟商标或注册式欧盟外观设计的修改,例如所有权信息和许可。
此外,EUIPO还向公众发布包括上述注册信息的《欧盟商标公告》和《欧盟外观设计公告》以及其他相关信息。
EUIPO不但致力于优化欧盟外观设计和欧盟商标的注册流程,还对孤儿作品数据库进行维护,提供会员国教育机构和档案库等公开渠道内的孤儿作品相关信息的访问渠道。
此外,EUIPO还负责监督知识产权侵权观察站,旨在通过鼓励良好的职业操守和合作规范来提高公众意识,从而协助打击假冒和盗版行为。自2012年起,EUIPO开始监督孤儿作品和侵权观察站。
EUIPO每年审查超15万件欧盟商标和9万件欧盟外观设计的申请,对促进欧盟地区的知识产权发展起着至关重要的作用。此外,EUIPO开展了全球范围内诸多国际合作和倡议,符合欧盟委员会对外政策的优先策略。例如,EUIPO致力于帮助人们认识到知识产权保护和注册的益处,鼓励创新,不断推进欧洲经济发展。
此外,EUIPO通过推进立法现代化和积极加入各类国际知识产权条约,推动知识产权管理和执法服务发展。通过谅解备忘录(MoU),EUIPO和其他知识产权局达成直接合作,确立了有利于欧盟成员国乃至全球知识产权领域的行动。
EUIPO还加入了商标五局(TM5)(商标5大知识产权局)和外观设计五局(ID5)(外观设计的5大知识产权局)。这5个知识产权局与世界知识产权组织(WIPO)保持着紧密的合作,分别是CNIPA(中国国家知识产权局)、EUIPO(欧盟知识产权局)、JPO(日本专利局)、KIPO(韩国知识产权局)和USPTO(美国专利商标局)。
EUIPO不仅仅是欧洲知识产权领域的焦点,也是其他地区知识产权保护的焦点。

委托书,通常缩写为POA,是一种法律文件,为一方代表或代理另一方处理某些事务或事项所出具的书面授权。此类事项包括法律诉讼、企业和商业事项,以及甚至个人事务,例如持久委托书(LPA).
具体到知识产权时,很多申请人和权利人选择,或基于适用的国家或地区知识产权法的规定,任命一位法律代表或代理作为代表申请人行事的第三方。
此类法律代表可以是专利代理人,例如,在相关国家或地区的知识产权局和/或法庭,就所委任的专利事项代表知识产权申请人或注册人。在这种情况下,委托书可作为代理人就申请人专利的申请和维持事项代表专利申请人的书面授权。
委托书也可用于在工作场所开发发明和专利相关的知识产权事务。当公司通过委托书来代表或获取员工在任职期间开发和创造的发明时,就会出现这种情况。
获得委托书以及提供用于知识产权事务的此类证明,各国要求都不一样。例如,针对委托书的使用,美国法律规定在本管辖区内,对获得委托书及其有效性有多项要求。
因此,在美国,一份针对法律实体的有效委托书应包括委托书签字日期、由委托人或在委托人在场并在其指示下有权签署文件的另一名成年人签字。此外,在美国,委托书必须在公证人面前签署确认或由两名证人签署。
除国内立法对获得委托书有所要求外,国家和地区法律还要求申请人和权利人遵守所提交或维持的知识产权所在知识产权局的规则。例如,在某些国家,委托书的代表或代理必须是知识产权提交或注册的管辖区内备案的代理人,但是这一规定并不适用于所有国家。
此外,很多管辖区要求提交一份文件,确认签署委托书的签字人可代表申请人就其知识产权获得申请日。一些国家还要求所提供的委托书必须为原件,且经公证和认证后才有效,但这并非适用于所有管辖区。
委托书是注册和维持知识产权的重要组成部分,往往也是至关重要的一部分。由于国际立法和要求有所不用,因此知识产权申请人和权利人应密切关注委托书签字和提交方面的必要要求,以便管理其知识产权组合。
.png)
管理专利注册全流程——从首次提交PCT申请到维持专利有效期长达20年甚至更久——可能是一个复杂且耗费资源的过程。IP-Coster通过提供集中化平台高效处理专利申请与保护的各个阶段,使这一流程得以简化。在我们的支持下,客户可专注于创新研发,而我们将为您统筹各项细节。
《专利合作条约》(PCT)允许申请人仅提交一件专利申请,随后可进入国家或地区阶段,在多达158个国家和地区获得保护。但是,PCT并不提供"国际专利",而是通过简化程序帮助申请人获得单一国家或地区专利。
传统PCT申请包含以下步骤:准备申请文件、获取所需签名、向受理局提交纸质副本。这一过程往往相当费时,特别是在需要协调法律代表以及处理大量文书工作的情况下。
IP-Coster 简化PCT专利申请
IP-Coster平台提供集中化、用户友好的解决方案,消除传统申请流程的障碍。我们的资深律师团队全程在线处理PCT申请,确保及时提交至全球89个受理局。所有沟通均通过数字化方式完成,无需纸质文件,显著降低行政负担。
进入国家或地区阶段
根据PCT规定,申请人需将申请转入国家或地区阶段,才能在各个管辖区获得专利保护。该步骤通常需在最早优先权日起30或31个月内完成(具体时限依各国/地区法规而定)。
此阶段需针对各个管辖区制定个性化策略。IP-Coster专家团队将协助客户优选目标市场,确保申请平稳过渡,显著提高专利授权成功率。
分地域独立管理专利申请流程
进入国家/地区阶段后,专利申请将在各管辖区独立进行。IP-Coster与全球可信赖的代理网络合作,为客户提供专业可靠的代理服务,确保符合当地法律和程序要求。
通过我们的平台,客户可在最初提交专利申请的同一直观界面上,无缝管理多个管辖区的知识产权事务——从申请到授权,全程获得专业护航。
维持专利有效性
专利授权后,需按时缴纳各国年费以维持专利有效性。这一持续性义务对保障专利权的稳定性至关重要。
IP-Coster提供高效的年费管理服务,既能简化行政流程,又可有效规避逾期风险,让客户在专注知识产权价值转化的同时,确保持续享有专利保护。
专利延期
针对医药、生物技术等特殊行业,为补偿合规审批耗时,专利可获得延期。根据具体标准及国家法规,最多可在20年基础保护期上延期5年。每件申请均需经过全面评估以确认延期的适用性。
IP-Coster资深专家团队将为您量身定制评估方案,为您争取专利延期,确保您的知识产权获得更长的保护期。